Describe a Specific Atom of an Element Using Isotope Notation.
Describe the general arrangement of subatomic particles in the atom. Atoms of the same element that have the same atomic number but different mass numbers.

Practice With Isotope Notation Youtube
Despite having different numbers of neutrons isotopes of the same element have very similar physical properties.
. Now write the isotopic notation for carbon-14. The atomic number also tells you the nuclear charge and the number of. You need to know this.
The atomic number is sometimes written as a subscript preceding the symbol but since this number defines the elements identity as does its symbol it is often omitted. Isotopes are atoms of the same element that have different numbers of neutrons but the same number of protons and electrons. Isotopic notation is used to describe a particular isotope of an element.
Electrons are found moving around the nucleus. Mass number - neutrons. So the mass number of element 5 6 11.
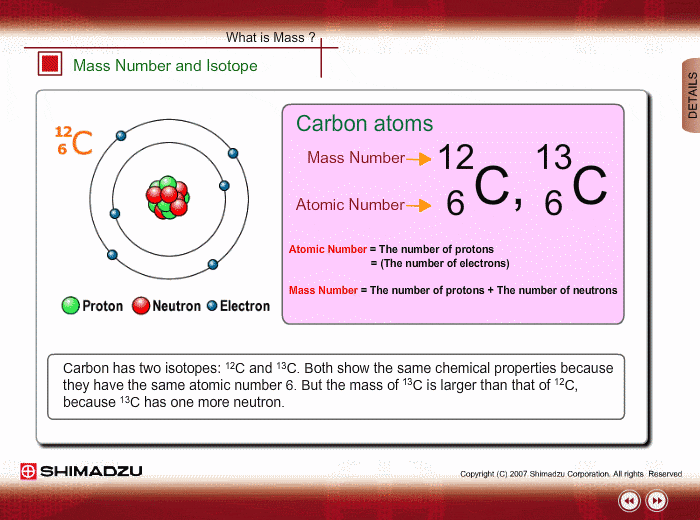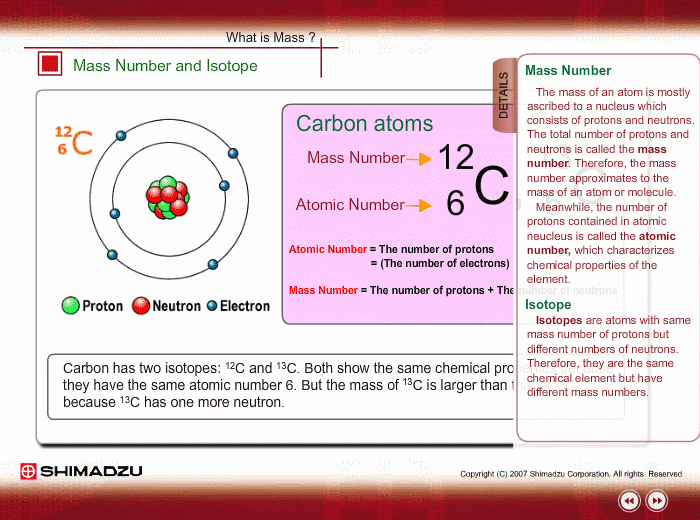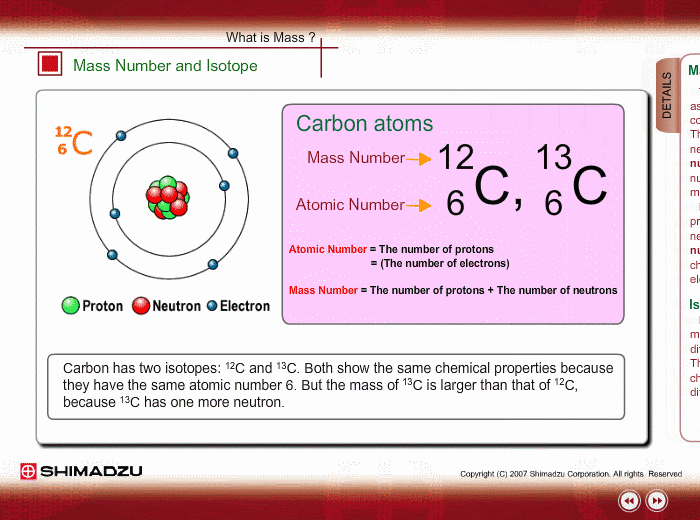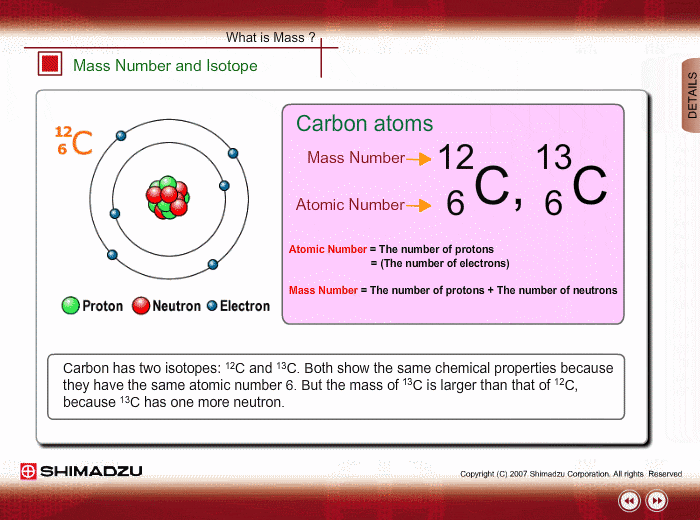
You might already know that each element has a symbol of its own eg Na for sodium Fe for iron O for Oxygen Li for lithium etc. The atomic number is equal to the number of protons in the nucleus. The particles found inside the nucleus are called _____ Mass number.
Same element with different number of neutrons. Mass number - protons. The name carbon-14 tells us that this isotopes mass number is 14.
Some isotopes are unstable and will undergo radioactive decay to become other elements. Click here to get an answer to your question What is the Isotope notation for a Lithium atom. Notation that shows the chemical symbol and mass number for an isotope of an element.
Consists of the ions element symbol and charge. Terms in this set 11 what is an isotope. Start studying Isotope notation.
Most of the mass of the atom comes from the nucleus. An element is identified by its atomic number. What is the isotopic notation for the isotope carbon-14.
An isotope with 6 protons and 7 neutrons is carbon-13 or C-16. The chemical symbol for carbon is C. List the mass number of an element after its name or element symbol.
Atoms and Isotopes Worksheet. Tap again to see term. From the periodic table we see that the atomic number number of protons for the element carbon is 6.
Um and that leads to the second type of notation. Learn vocabulary terms and more with flashcards games and other study tools. 1 See answer ChrisGibson294 is waiting for your help.
Same as atomic number. The number of protons and neutrons combined is called the _____ Less. So um the isotope of a particular element can be determined by subtracting the atomic number from the mass number um which gives the number of neutrons in the isotope.
The superscript number to the left of the element abbreviation indicates the number of protons plus neutrons in the isotope. The type of element. Charge Electrons.
The identity of an atom is determined by the number of _____ Protons and neutrons. From this you can determine various attributes of that specific atom of the element. It is also called the nuclide symbol.
The symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol see image below. Click again to see term. The average mass of all the isotopes of an element.
The representation of a specific isotope. Isotopes are elements that have the same atomic number with a different mass number. A mass number.
Note the mass number of two isotopes may be the same even though they are different elements. Fill in the table with the correct information. The isotopic symbol is literally the symbol of isotopes.
Number of protons number of neutrons. What is the isotope notation of the element that has an atomic number of. And then um it also gives this notation also gives the mass number which is the sum of the number of protons and neutrons.
Describe the general arrangement of subatomic particles in the atom. Tap card to see definition. Isotopes are atoms of the same element that contain an identical number of protons but a different number of neutrons.
Isotopes and isotope notation. The atomic number is listed below the symbol the mass number above. ChrisGibson294 ChrisGibson294 05022017 Physics High School answered What is the Isotope notation for a Lithium atom.
Isotope Isotope Notation Atomic Protons Electrons Neutrons. Z atomic number. The following chart summarizes the subatomic particles that make up an atom.
Have different numbers of. The total number of protons and neutrons in an atom. The following element notation X symbol of element.
For example an isotope with 6 protons and 6 neutrons is carbon-12 or C-12. Add your answer and earn points. Click card to see definition.
Number of protons number of electrons on neutral elements.

Comments
Post a Comment